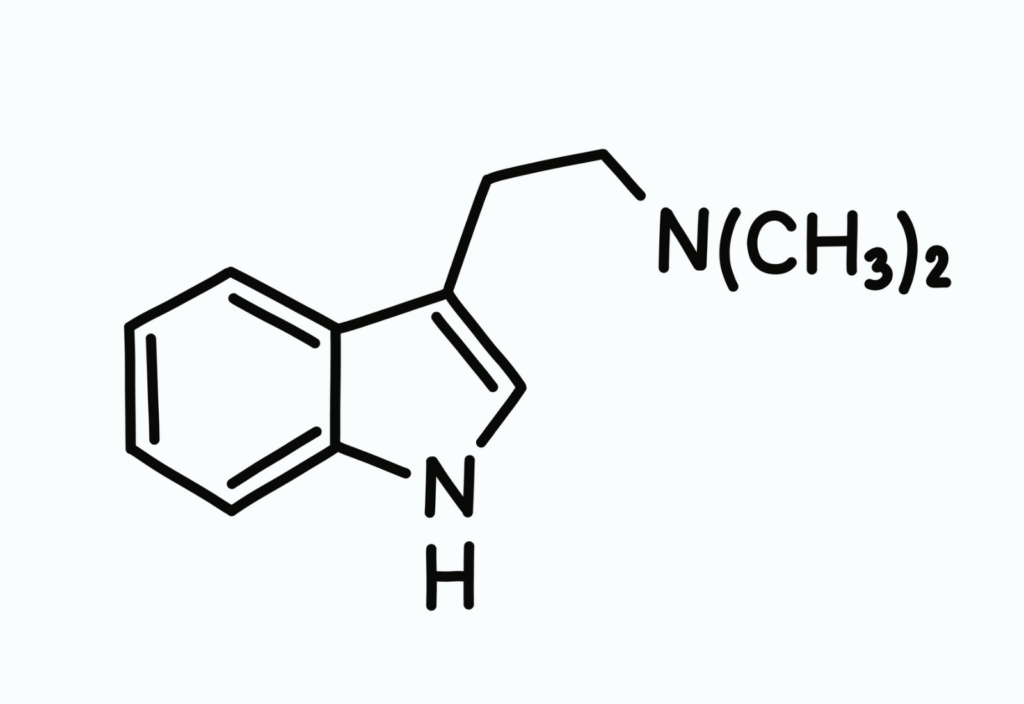
DMT, or N, N-dimethyltryptamine is a powerful psychedelic. DMT, like its sister psychedelics (LSD, MDMA, psilocybin … and even marijuana), is a Schedule I controlled substance. So according to the DEA, its abuse potential is high and it has no medical use. Also, according to the DEA, “the history of human experience probably goes back several hundred years since DMT usage is associated with a number of religious practices and rituals.” Indeed, DMT is the active ingredient in ayahuasca and has been used by indigenous communities in the Amazon for centuries; here, the past few decades have seen a resurgence in the use of DMT and/or ayahuasca ceremonies by non-indigenous persons.
Notwithstanding its dubious distinction as a Schedule I drug, DMT increasingly is the subject of study for its potential efficacy in treating a wide array of mental-health conditions including depression, anxiety, and PTSD. This research—as with psilocybin, MDMA, ketamine, ibogaine etc.—is expanding rapidly as numerous neuropharmaceutical companies look to be among the first-to-market potentially revolutionary therapies.
Just last month, Small Pharma, a neuropharmaceutical company, announced that that it has been granted an Innovation Passport Designation by the U.K. Medicines and Healthcare Products Regulatory Agency for one of its DMT products for the treatment of major depressive disorder. This designation is similar to the FDA’s fast-track approval process in the United States. In fact, Small Pharma has been conducting trials as reported by the BBC and expanded its clinical trial this past summer.
In September, the Nikean Foundation announced a $5 million gift to create a psychedelic therapy research center at the University of Toronto. Several months ago, Canada-based Algernon Pharmaceuticals submitted a request to U.S. regulators to launch a clinical program to study the use of DMT for the treatment of stroke-related dysfunction. Last July, researchers published a study of the use of DMT to treat U.S. Special Forces Operations Veterans with promising results. And of course, the Multidisciplinary Association for Psychedelic Studies (“MAPS”) has been leading the charge since 1986. MAPS completed a study on DMT back in 2013 working with the renowned physician and author, Gabor Mate, whose work on recovering from trauma (see In the Realm of Hungry Ghosts: Close Encounters With Addiction) is essential reading.
But the pathways from research and clinical trials to the actual use of DMT and other psychedelics as treatment modalities is a winding one. As our own Mason Marks wrote in a recent co-authored article for Nature, Psychedelic therapy: a roadmap for wider acceptance and utilization, the Schedule I status of psychedelics means that federal funding for research is virtually non-existent. Other barriers to use include the rush to patent various compounds as this may limit access to these emerging therapies. And of course changing public perception toward accepting the use of psychedelics after five decades of a campaign to vilify any and all use.
Oregon is embarking on a grand experiment when it comes to psilocybin; one that may have seemed impossible even five years ago. So perhaps the use of DMT and other psychedelics for treating America’s mental health crisis is not far off. In the near term, however, it appears other countries will outpace the United States by leaps and bounds because of our federal government’s refusal to end the failed War on Drugs. So perhaps the skyrocketing “medical tourism” trade coming from the United States will soon include “mental health tourism”.
For additional reading on psychedelics, microdosing, and related topics, see:
- Here’s How MDMA Will be Regulated
- Dispatches from Wonderland Miami
- Is Psilocybin Really a Miracle Drug? Compass Pathways Releases Phase 2B Trial Results
- A Strategy for Rescheduling Psilocybin
- BREAKING: City of Seattle Decriminalizes Psychedelics
- California Psychedelic Law Update
- The Psychedelic Landscape: Pharma, Tech, Decrim, Other
- Psychedelics Legislation Advances in California
- MDMA (Ecstasy) Nears FDA Approval for PTSD Treatment
- Psychedelic Decriminalization and Legalization Roundup
- Another Win for Religious Use of Psychedelics: New Hampshire v. Mack