A common question that arises in Ketamine clinic transactions is whether a clinic or physician (collectively, “healthcare providers” or “providers”) can use or promote Ketamine for off-label uses. The short answer is yes, subject to several caveats, as discussed below. However, before reaching the answer, it is important to define what “off-label” use means and whether the U.S. Food and Drug Administration (“FDA”) has jurisdiction over the healthcare providers to enforce off-label promotion.
What is “Off-Label” Use?
The term “off-label” means that a prescription is being used for an indication that is not set forth on the drug’s label. Labels must be approved by the FDA as part of the pre-market approval process and must contain certain information about the approved uses. See, e.g., 21 C.F.R. § 201.56(a)(3) (“The labeling must be based whenever possible on data derived from human experience.”). The label is prepared after, among other things, clinical trials have been completed and approved by the FDA. See, e.g., 21 U.S.C. § 505(i) (requiring clinical trials before a drug can be approved for marketing).
The FDA may decline a new drug application when, for example, the evidence fails to demonstrate the drug’s safety or “there is a lack of substantial evidence that the drug will have the” claimed effect. 21 U.S.C. § 355(d). (“‘[S]ubstantial evidence’ means evidence consisting of adequate and well-controlled investigations, including clinical investigations, by experts qualified by scientific training and experience to evaluate the effectiveness of the drug involved, on the basis of which it could fairly and responsibly be concluded by such experts that the drug will have the effect it purports or is represented to have under the [proposed] conditions of use….”).
The Federal Food, Drug, and Cosmetic Act (“FDCA”) (codified at 21 U.S.C. § 301 et seq.) and FDA regulations generally prohibit manufacturers from marketing, advertising, or otherwise promoting drugs for unapproved or “off-label” uses. See 21 U.S.C. §§ 331(a) & (d) (prohibiting manufacturers from introducing misbranded or unapproved drugs into interstate commerce); see also, e.g., 21 C.F.R. § 202.1(e)(4)(i)(a) (“An advertisement for a prescription drug …shall not recommend or suggest any use that is not in the labeling accepted in [the] approved new-drug application….”).
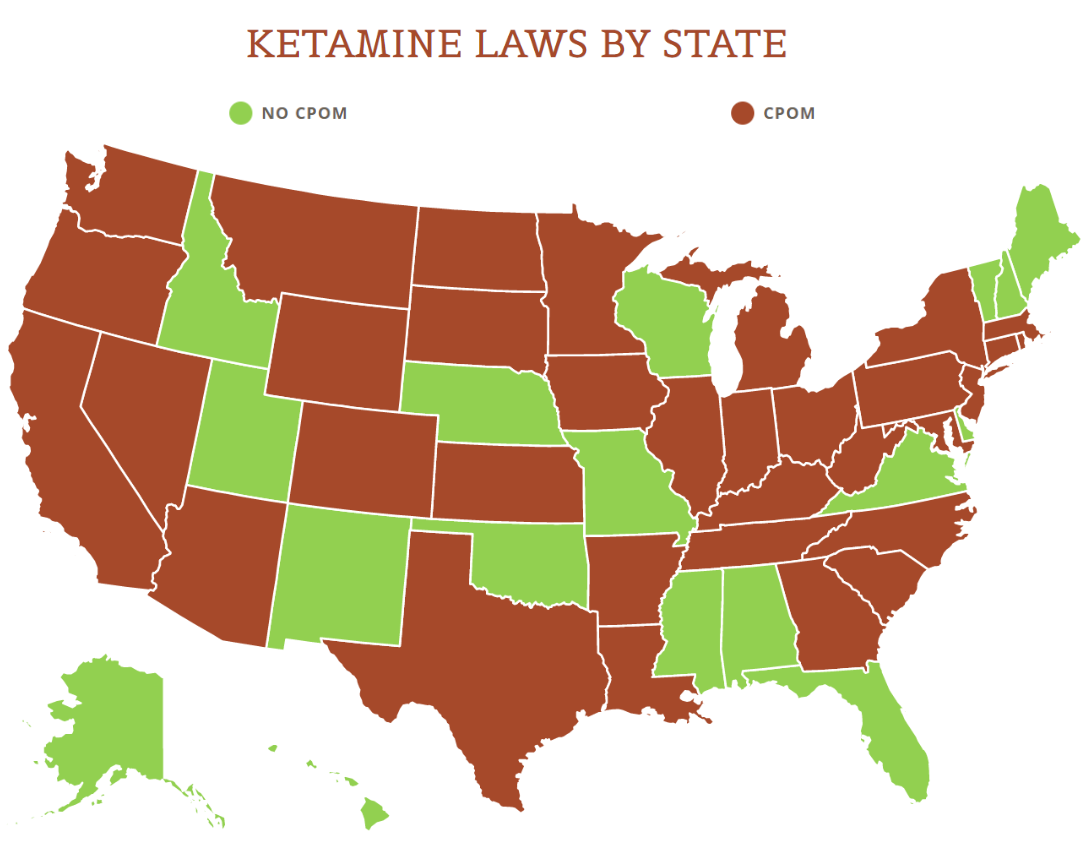
View the US Map of Ketamine Legality
Are Healthcare Providers Prohibited from Using Drugs Off-Label?
While there are very strict laws and regulations about off-label promotion for drug manufacturers, healthcare providers are not subject to those same restrictions (if such providers are not employed by or contracted with a pharmaceutical manufacturer). See Dresser R, Frader J, Off-Label Prescribing: A Call for Heightened Professional and Government Oversight, 37 J. Law Med. Ethics 476 (2009) (“Consistent with its jurisdictional authority, the FDA controls manufacturers’ product marketing.”). A healthcare provider has the absolute right to discuss and recommend drugs for off-label uses with patients. Buckman Co. v. Plaintiffs’ Legal Comm., 531 U.S. 341, 350–51 (2001) (because the FDCA does not regulate the practice of medicine, and because prescription drugs may have therapeutic uses other than their FDA-approved indications, physicians may lawfully prescribe drugs for off-label use); see also Teo W: FDA and the practice of medicine: looking at off-label drugs, 41 Seton Hall Legis. J, 305, 307 (2016) (the FDA has consistently maintained that “it does not regulate the practice of medicine between physicians and patients.” (footnote omitted)).
What Conditions Is Ketamine Approved For?
Ketamine is a Schedule III non-narcotic controlled substance in the United States pursuant to the Federal Comprehensive Drug Abuse Prevention and Control Act of 1970, which means it is a lawful prescription drug that has accepted medical use. 21 C.F.R. § 1308.13. Ketamine is typically used as a surgical anesthetic and has been approved for the treatment of certain types of depression. See Prescriber’s Digital Reference (2021), https://www.pdr.net/ (ketamine hydrochloride is approved for induction and maintenance of anesthesia and procedural sedation, and esketamine hydrochloride is approved for the treatment of depression in conjunction with an oral antidepressant and for the treatment of treatment-resistant depression in adults). Therefore, the use of ketamine for any other conditions (e.g., pain relief) is considered off-label. However, physicians are increasingly using ketamine in the off-label capacity to treat mood disorders, pain symptoms, and other conditions.
Can Providers Use and Promote Ketamine for Any Condition?
While the FDA may not have jurisdiction over healthcare providers for off-label uses, that does not mean a provider can market (or make “claims”) ketamine for any condition without fear of reprisal. There are two primary legal areas that a healthcare provider should be aware of – medical malpractice claims and deceptive trade practice claims under state and federal law (under federal law, the Federal Trade Commission has jurisdiction to bring an action against a provider).
Using and promoting ketamine for a condition that is not “on-label” can lead to malpractice claims against unwary healthcare providers. A malpractice claim often revolves around whether the physician’s care met the community standard for treating a specific condition. In the absence of FDA approval or extensive research that supports the use of a drug for a particular condition, a provider may have a difficult time convincing a jury that her care was consistent with the community standard (click here for a summary of possible bases for malpractice claims, which notes –“Plaintiffs may recover in off-label medical malpractice cases if it can be established that a physician’s off-label prescription deviated from an acceptable and prevailing standard of practice.”).
The second area of concern is whether the off-label promotion is deceptive to consumers. There are both state and federal laws that are intended to protect consumers from false or misleading advertising. Click here for a summary of the Unfair and Deceptive Acts and Practices (UDAP) statutes in each of the fifty states and the District of Columbia.
Under the deceptive trade practices theory, if a healthcare provider makes “claims” about a drug that have no clinical evidence to support such claims, it is easy to see how this could lead to a deceptive trade practices action when a drug does not work as advertised (or worse yet, injures the patient). For example, if a provider posted on her website that one injection of ketamine was a “cure” or “100% effective” for the treatment of psychosis, that could certainly help form the basis of a deceptive claim action.
Thus, care must be taken to promote off-label uses of ketamine in a non-misleading way. The gold standard for “proving” a “claim” is a well-controlled randomized clinical trial that is statistically significant (e.g., the results can be generalized beyond the research and control groups). Certainly, FDA approval requires such proof, and in the absence of FDA approval, other credible evidence must exist to insulate healthcare providers from various legal actions.