Over the past few years, our international trade team has advised a rapidly expanding suite of clients on import/export issues related to cannabis. We regularly advise clients on manufacturing overseas, hemp import/export issues, and customs. Currently, we are also working on heady questions surrounding international treaties and cannabis. One thing no one has hired us for yet, though–and which I would love to work on–is shipping medical cannabis internationally.
Can Marijuana Be Shipped Internationally?
The straight answer is — possibly when you follow the right procedure. So, how do you ship medical cannabis internationally?
Here are the steps:
1. Start in a country with federal laws allowing medical cannabis production.
There are quite a few countries worldwide interested in cannabis product imports. Of course, exports will always be driven by demand. And demand is not solely a matter of quantity; product categories are also dispositive. To date we have seen medical cannabis import/export in categories including whole flower, oil, topicals and capsules. Some of this cannabis has been exported for research purposes, but the majority seems to have shipped for medical application. This is generally because the importing countries allow medical marijuana or cannabis consumption, but do not license production and do not tolerate home grow.
2. Find a country with a progressive national health department and importing/exporting authority.
Any of Canada, the Netherlands, Uruguay, Colombia, Israel, Jamaica, South Africa, Lesotho or Australia would probably suffice. The country you select must also be one that allows medical cannabis imports. There are quite a few, especially in the E.U.
3. Hire an experienced international trade law attorney.
Exporting cannabis is a complex process to navigate. First, you must determine the permissibility of the export under existing controls. Such an endeavor generally requires working with an attorney experienced in international trade laws. When the product doesn’t appear on the Commerce Control List (CCL) — a set of standards determining whether an item can be shipped and where — a knowledgeable professional can help you move forward.
Articles on the CCL will have an Export Control Classification Number (ECCN) based on their characteristics, which determines whether exporting them requires a license. In the absence of an ECCN, items are designated as EAR99, which means exporting them does not call for specific licensure.
Items assigned an ECCN generally fall into categories such as electronics or technology, but it is still worthwhile to have an experienced legal expert review your product to ensure an ECCN does not apply. At that point, you can have your professional obtain a Commodity Classification Automated Tracking System (CCATS) number from the Bureau of Industry and Security (BIS) to give the product a formal classification.
4. Strike a deal and acquire import and export permits.
Although not every circumstance requires it, a CCATS may provide assurances to finance partners, customs brokers and receiving entities by demonstrating that you went through the proper channels to export your product. That means it may be easier to find a suitable consignee in your destination and get the necessary permits.
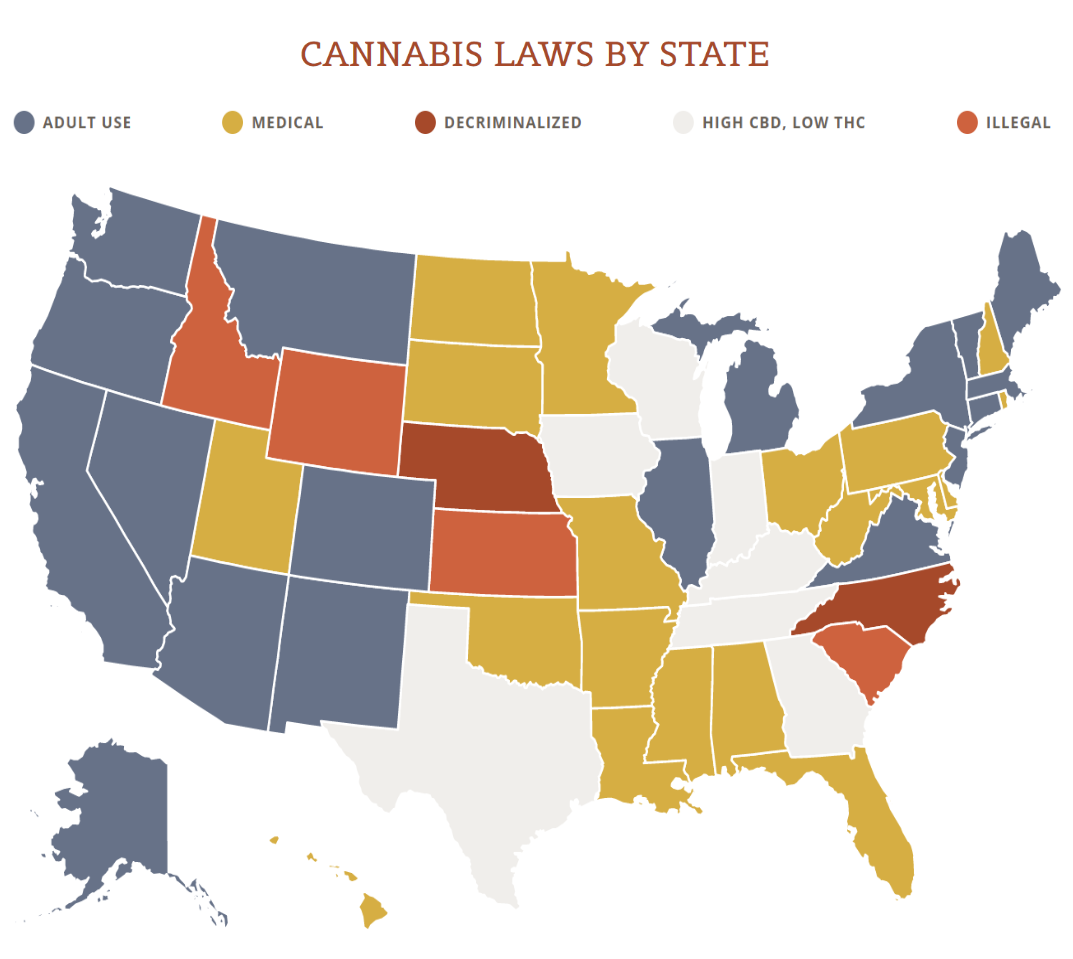
View the US Map of Marijuana Legality
5. Carry out due diligence.
After obtaining a CCATS, exporters should conduct due diligence on any partner in the transaction and the ultimate product destination. Due to embargoes, export to Cuba, North Korea, Syria and Iran are impermissible except by explicit licensing from appropriate U.S. agencies. All parties to the transaction — including entities and their majority stakeholders — should also clear the restricted-parties lists with the BIS and the Office of Foreign Assets Control (OFAC).
6. Report all imports and exports to the U.S. Census Bureau and International Narcotics Control Board (INCB) as required.
When shipment values exceed a specific monetary threshold, exporters must electronically file their exporting information with the U.S. Census Bureau, which provides all the information about classification, recipient and destination. OFAC and BIS use this data to enforce export controls.
The INCB is the United Nations’ independent control body for international drug conventions. In the context of cannabis, the 1961 Single Convention allows cannabis to be produced and administered for medical and research purposes under certain conditions. The required controls include that a government agency designates the area where cannabis can be cultivated, licenses producers, and has the exclusive right to import, export, trade at wholesale, and maintain supply. Each of the export countries listed above has taken steps along these lines.
Practical Matters
The medical cannabis import/export market is very new. This means that aside from the legal complexities, there are practical matters to work through.
Foremost among these are quality standards. Although good manufacturing practice (GMP) adherence is required to ship medical cannabis to the E.U., for example, there are no standardized regulations between and among countries for medical cannabis quality control— including for content, composition, adulterants, potency and even levels of toxic residues. (Think about that… for a “medicine”!)
Another critical issue is supply chain integrity. Cannabis consumers — especially those seeking medical relief — likely want assurances of the product’s origin and composition. Companies will only be able to deliver on those expectations with transparency, documentation and quality control as cornerstones of their supply chains.
Finally, a thicket of political and policy considerations must be navigated, extending to social responsibility and end-user frameworks. Companies engaging in cannabis export face heightened scrutiny from both foreign and domestic agencies, not to mention state law disparities that can disrupt transportation across domestic borders. Even with comprehensive documentation, challenges can and do arise.
Firms pressing into the medical cannabis exporting space typically describe their efforts as long-term investments rather than one-off projects. This makes sense given the capital requirements for entry and the political wherewithal needed to gain international footing. Indeed, the quantities of cannabis shipped will be sizable and spaced at irregular intervals: this is not an area for dabblers.
Ultimately, exporting medical cannabis from places where it grows best, like Colombia, to places it might not, like England, seems natural – just as it does for Colombia to grow and send coffee overseas. Today, Canada has a big head start on export; however, one wonders whether it makes sense for cannabis to be grown north of the 42nd parallel long-term. At some point, the legal regime will settle out and market efficiencies may follow.
In all, legal and political factors that once made medical marijuana export unthinkable are changing, and changing very fast. The international distribution channels being built today will one day serve as conduits for the recreational cannabis trade as well. Until then, we will continue to monitor and report on developments in this fascinating space.
For more on the international cannabis trade, check out the following:
- International Cannabis: Selling Worldwide
- U.S. Cannabis and International Trade: Never the Twain Shall Meet?
- International Cannabis: Breaking the Law, Staying Honest
- Should You Import Hemp to the United States?
- Border Woes: Transporting CBD into Canada is Not OK
- Cannabis and International Trade: Don’t Ignore the U.S.–China Trade War
- Cannabis, Tariffs and Vaping Imports from China
- Cannabis Legalization Roundup: Mexico, Luxembourg, Switzerland
Bear in mind that the information provided here is primarily focused on the legalities and procedures involved in exporting goods and does not cover all of the regulations surrounding cannabis products. It does not constitute legal advice.
For more specific guidance on THC export, reach out to Harris Sliwoski.