On June 14, 2022, Harris Sliwoski attorneys Fred Rocafort, Jihee Ahn, Paul Coble, and Vincent Silwoski presented a webinar entitled Protecting, Monetizing and Enforcing Cannabis Intellectual Property. Attendees submitted many great questions before and during the webinar, but our IP attorneys were not able to answer all of them. In this post, we will answer the cannabis patent questions that were not addressed in the webinar.
What do you think about using the Plant Variety Protection Act to protect strains?
The Plant Variety Protection Act (PVPA) can provide protection for both asexually and sexually reproducing plants, like cannabis. The pseudo-patent protection available under the PVPA prohibits other from marketing, selling, delivering, exchanging, transferring, or multiplying a protected strain. However, current practical realities make PVPA protection unavailable for most cannabis patent strains. The PVPA includes a strict requirement that at least 3,000 seeds of the claimed plant species be deposited with the U.S. Department of Agriculture in Fort Collins, CO. The USDA will not accept any deposits for plants that are classified as controlled substances, including cannabis. In other words, for the time being, PVPA protection is unavailable for cannabis plants that do not qualify as hemp (less than 0.3% delta-9-THC). The DEA recently stated that cannabis seeds containing less than 0.3% d9-THC are not controlled substances regardless of the THC content of the mature plant, but it is not yet clear whether the DEA’s statement will affect the USDA’s prohibition.
How can I protect a recipe/formulation that is unique to the market? How do I present a Fast-Acting ingredient and protect the recipe? How do I sell license opportunities for my recipes?
New and useful compounds, as well as novel formulations of known compounds, can be protected with a utility cannabis patent. Licensing strategy is a complex issue that depends on the nature of the invention and inventor(s), but obtaining a patent almost always strengthens licensing leverage. Sometimes just having a patent application on file is enough to license rights to larger companies or patent monetization firms.
Is it possible to obtain a cannabis patent for a methodology that cures or eliminates a cannabis pathogen?
Absolutely, so long as it is new, useful, and has not been disclosed or used in public (some exceptions may apply). You will also need to be able to describe the methodology in sufficient detail such that a typical cannabis cultivator can achieve the desired outcome without undue experimentation.
Who owns patentable IP that is jointly developed by multiple parties?
Absent an enforceable agreement addressing IP ownership, patent rights in the US vest by default with the inventors. Any person who materially contributed to the claims of the patent must be listed as an inventor and the inventors each have an individual right to practice and license the patent. If the inventors work for different companies, ownership of the IP will be dictated by any agreements the inventors have with their employers.
I’d like to hear thoughts on enforcement of patents on cannabis paraphernalia, specifically electronic devices and whether there’s any reason to believe there are any more issues with those than with enforcement of patents on any other consumer good.
Patents on cannabis paraphernalia (namely vaporizers, water pipes, rolling papers, etc.) are a common subject for both utility and design patents. Electronic vaporizers and cartridges are one of the most common cannabis patents, with several new patents issuing each week. There is no reason to believe that such patents are any less enforceable than any other patent, but none have been tested in court.
Ramifications of Canopy vs GW Pharma?
For the uninitiated, Canopy Growth v. GW Pharmaceuticals et al., Case No. 6:20-cv-01180-ADA (W.D. Tex.), is one of the first cannabis patent infringement cases in the US and sets the stage for many more to come. In late 2019, Canopy Growth obtained a patent for subcritical CO2 extraction and promptly sued GW Pharma in Texas federal court. As part of every patent infringement lawsuit, the court is required to construe the claims of the patent and, where necessary, put the patent language into terms that can be applied to the allegedly infringing product or process. Canopy and GW agreed on the meaning of all claim terms, except for one. GW argued that the patent term “CO2 in liquefied form under subcritical pressure and temperature conditions” meant that both the temperature and pressure had to be below the critical point to infringe (i.e. only extraction conditions in the red portion below):
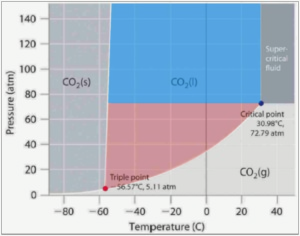
Canopy contended that liquified CO2 at either a lower pressure or a lower temperature than the critical point would constitute subcritical conditions (i.e. the red and blue portions). The court agreed with GW. Since GW’s processes are presumably solely within the blue range excluded by the court’s construction, Canopy consented to judgment of non-infringement and promptly appealed.
For now, there will be few direct ramifications from the case for most operators. Extractors that are operating processes with extraction conditions in the red range should consult a patent attorney to discuss their options. More broadly, however, it serves as an indicator that major cannabis patent suits are coming and highlights the importance of understanding cannabis science in cannabis patent analysis.